It is a very important societal need to understand the impact of using various surfactant-based formulations (conventional or biosurfactants) for personal care and health care on us, or why products or processes work the way they do and the applications of these processes.
Surface Active agent or Surfactants form a unique class of simple chemical compounds that lower the surface tension (or interfacial tension) between two liquids, between a gas and a liquid, or between a liquid and a solid. Surfactants touch our everyday lives in countless ways. They are present in our food, our drinks, and the products that we use to clean ourselves (e.g., soaps, shampoos, toothpaste, shower gel) clean clothes (detergents), cosmetics that we apply (e.g., creams, lotions, gels, perfumes), the medicines that we take (syrups, tablets, injections) just to name a few ways. Surfactants are usually an organic amphiphilic substance containing hydrophilic, polar or ionic, head group and hydrophobic, non-polar chain.
In the bulk aqueous phase, surfactants form aggregates, such as spherical micelles (low viscosity) or cylindrical micelles (high viscosity) depending on the chemical structure of the surfactants. Supramolecular nanostructures (self-assembly) formed by these surfactants display exceptional advantages because of the flexible and tunable nature. The universal structure of a surfactant molecule and various self-assembled structures are shown in the figure.
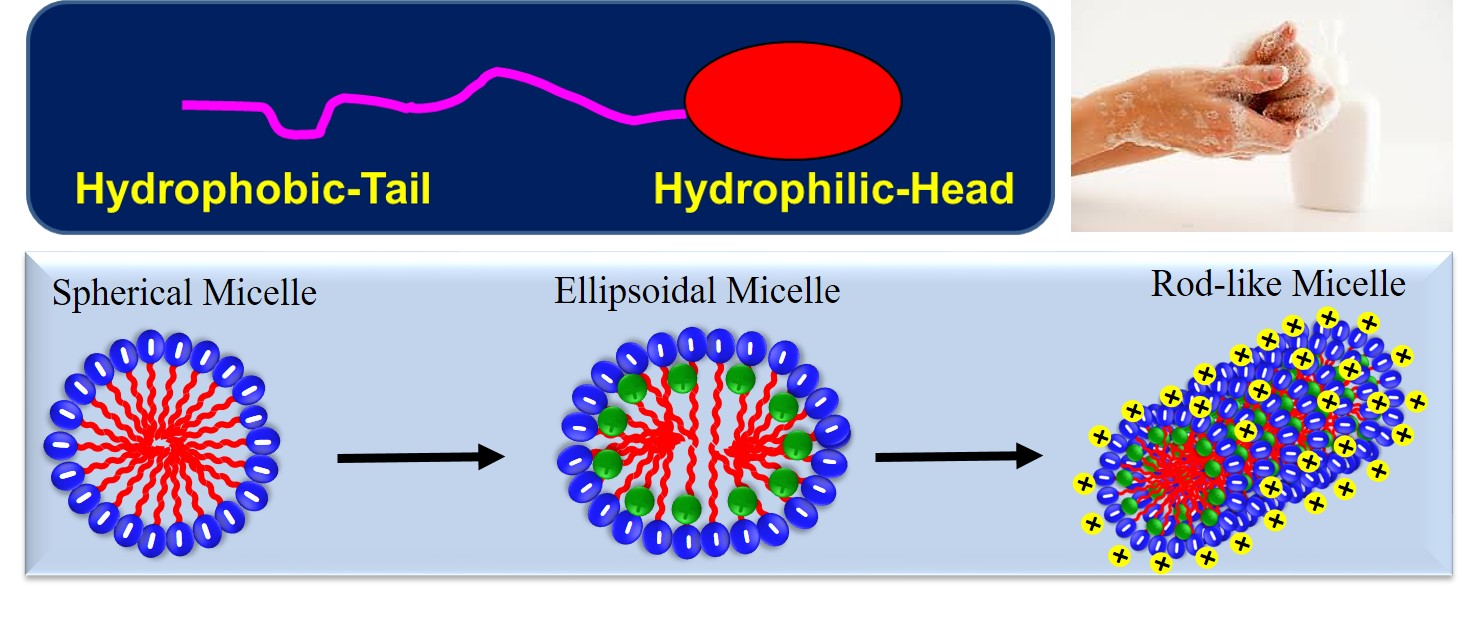
Biosurfactants are fetching a smart alternative for conventional surfactants. This evolution is gaining prominence as product developmental exertions in application areas such as cosmetics, personal care, and agriculture now expect substantial outcomes after shifting toward non-crude-based surfactants. There is also a noteworthy impetus towards eco-friendly choices across the globe, owing to prompt modifications in guidelines around material use. These are assisting Research & Development efforts towards biosurfactants.
In my research group, we have developed a profound understanding and built a tailored approach for the utilisation of green surfactants in mixtures for various cosmetic product formulations working in close collaboration with industry on:
• By what means do molecular structures of surfactants and additives influence their solution, interfacial and colloidal properties?
• Exactly how do these properties affect their mechanism in formation of foams, emulsion, microemulsion and rod-like micelles?
• In what way one can select surfactants for optimal performance in practical applications?
• By what method do we make liquid foams with superior foamability and foam stability?
• How can you design a surfactant formulation depicting unique viscoelastic behaviour?
I also have a strong interest in teaching basic chemistry, including surfactant and polymer courses. Teaching in these areas is the natural extension of my involvement in this research field, and has led to a series of publications on the structural properties and characteristics of the systems. At Ahmedabad University, I have developed an elective course on surfactant science and nanotechnology based on my research activities. My exploration permits me to dig deep into the core of surfactant science, which focuses primarily on basic surface chemistry principles to recognise the structure and reactivity of surfactants i.e. on the formation and characterisation of the self-assembly structures of surfactant molecules.


